Insights from the Italian patient registry

Hello Glut1 Community!
Happy Spring to everyone!!
For this month’s post I would like to highlight a recent publication by Varesio C, et al. in Orphanet Journal of Rare diseases, based on an Italian multicenter collaborative project promoted and coordinated by the Italian GLUT1DS Association. The title of the publication is: GLUT1-DS Italian registry: past, present, and future: a useful tool for rare disorders.
The objective:
To describe the implementation of a national web-based registry for GLUT1 Deficiency.
The methods:
This was a retrospective study, which is a study that looks at events that have taken place in the past, and a prospective study, which is a study that follows individuals over time in the future. This study was also an observational and multicenter (5 Italian centers) registry developed in collaboration with the Italian GLUT1-DS association and was custom built on a cloud computing technology platform called cloud-R. Their registry collects baseline and follow-up data on the patient’s demographics, history, symptoms, genotype, clinical, and instrumental evaluations and therapies. This registry is a longitudinal study, which means that it tracks the same type of information on the same patients at multiple time points.
This registry is owned by the Italian GLUT1DS association and the coordination is entrusted to a steering committee composed of a Scientific Advisory Board represented by expert clinicians and Italian patient representatives. In addition, they have an external advisor and a registry manager.
Participation in the registry is voluntary and their consent allows the registry team to access medical records and test results, to contact patients over time and to include the data in scientific publications. Only clinicians or the registry manager can input the data.
Who took part in the study?
Individuals with:
- Confirmed Glut1 Deficiency diagnosis based on genetic testing and/or lumbar puncture tests
- Possible diagnosis, with symptoms but not a confirmed diagnosis by lumbar puncture, however there was an SLC2A1 gene variant present
- Uncertain diagnosis for patients with symptoms that suggest Glut1 Deficiency, with positive lumbar puncture test but no SLC2A1 gene variant present or deletion in the gene
- Patients with symptoms suggesting Glut1 deficiency without a positive lumbar puncture test or genetic testing were not included in the study
The Results:
Some of the data that is included in this study are follow-up visits, blood tests, cerebrospinal fluid tests, diet therapies and other types of therapies.
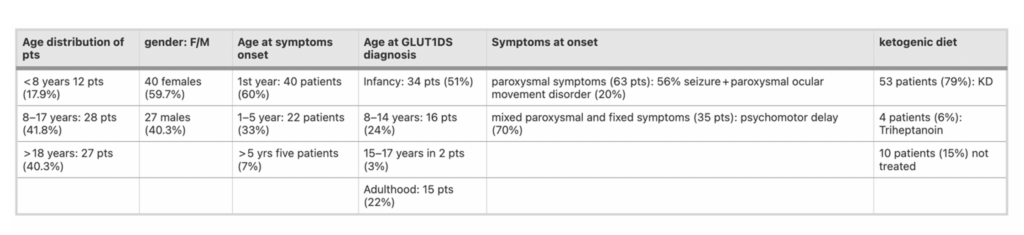
Bellow you can see a table with the summary with some clinical data collected from patients.
Varesio, C et al 2023
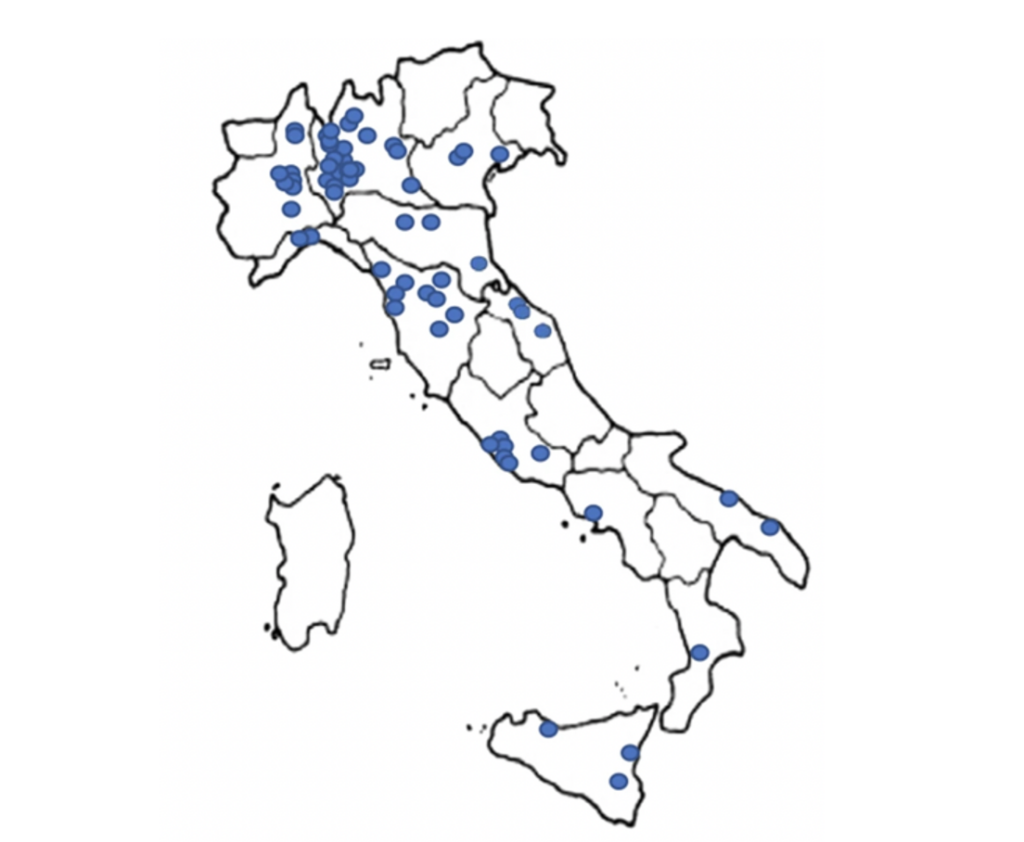
Geographical distribution of patients in the Italian Glut1 Deficiency registry
Varesio, C et al 2023
The Discussion:
This study emphasizes the important role patients play in this and other studies with the goal to follow a group of people over time who have a specific disease, collecting health information in order to understand how the disease develops and changes and how to diagnose and treat it better. Similar to our own Natural History Study, the Italian registry wants to collect patient data over time to later describe the natural history of the disease. The difference between the two studies is that the Italian study collects only clinical data from clinical centers enrolled in the Italian registry and patients do not input any data. On the other hand, at this time our Matrix Natural History Study is based on patient reported data and later it will include centers of excellence that like in the Italian study, will collect clinician entered data. Additionally, we have just formed a new partnership with the Rare Patient Network powered by Ciitizen, which will allow the collection of health records which will be organized and analyzed to help inform a better understanding of Glut1 Deficiency. Patients can upload these records to our Matrix Natural History Study and in this way, we can have a more complete information from our patient community.
One of the aspects the team in Italy points out is the design of their registry to have common data elements that allows it to communicate with other databases and registries and ensure some level of international interoperability or ability to exchange data between registries. This is important because this characteristic could possibly allow the establishment of a more robust Glut1 Deficiency Natural History Study to generate a more comprehensive patient information database. Each database separately has the potential to help identify biomarkers for this disease and to establish outcome measures for future clinical studies; however, a combination of both studies, the Italian registry and our Natural History Study would be even more informative for researchers and clinicians to have a better understanding of the disease and to pursue the development of better treatments and eventually a cure.
We look forward to collaborating with the Italian patient and research community on these efforts as our work moves forward and appreciate the excellent model they’ve created, all the work and dedication they have invested already, and the insights they have made available through the publication.



